Does the piglet gut health depend on the type and level of dietary fiber?
The intestine, besides digesting and absorbing nutrients from food, is responsible for the protection against pathogenic organisms and toxins. A healthy intestine is provided with:
A diet with sufficient and adequate nutrients.
A mucosa (composed of the epithelium, lymphoid tissue, and mucus that covers the epithelium) that maintains intestinal integrity.
A microbiota (bacteria, archaea, protists, fungi, and viruses) that maintains intestinal homeostasis.
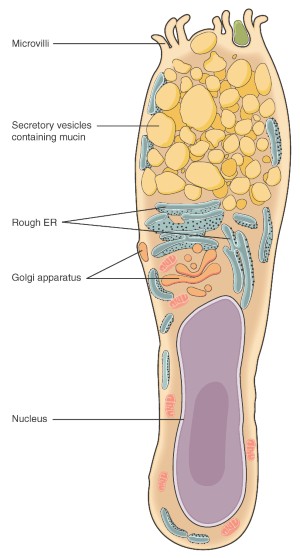
An immune system, both innate and adaptive, composed of hematopoietic cells (macrophages, dendritic cells, and T cells) and non-hematopoietic cells (epithelial cells, Paneth cells [1] and goblet cells) that maintain a balanced intestinal environment [2] . It should be studied and considered from a holistic point of view as can be seen in figure 1 (modified from Jha, R et al., 2019).
Figure 1: Holistic approach of the intestinal health and its components.
The intestinal mucus, the intestinal epithelial cells, the lymphoid tissue and the microbiota interact to form a fragile and dynamic balance, critical for an efficient digestive absorption and functionality.
Dietary fiber
Dietary fiber is the set of carbohydrates that cannot be digested by digestive enzymes. Chemically, it can be defined as the sum of non-starch polysaccharides and lignin. Hoffman in 2013 [3], characterizes the composition of carbohydrates in a diet according to the following figure:
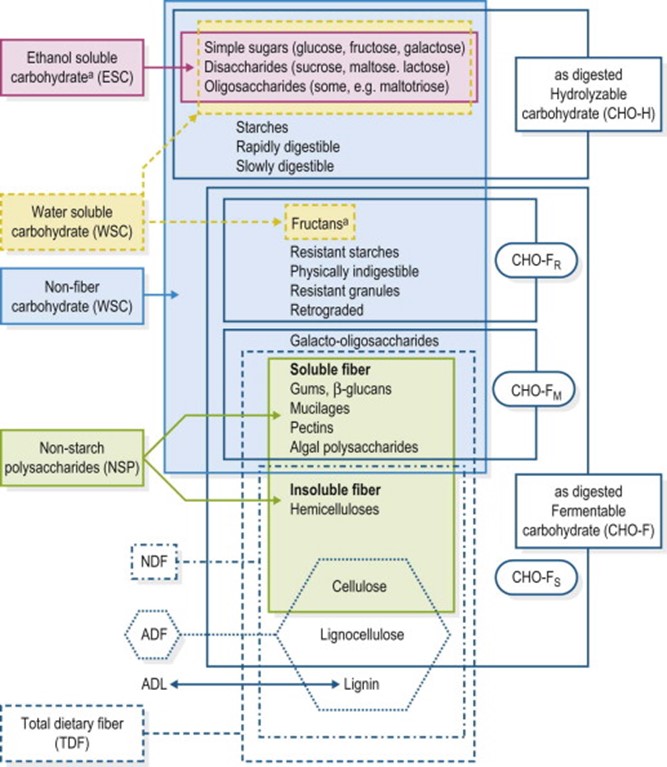
In pigs, dietary fiber is used depending on the degree of fermentation suffered by the microbiota in the small and large intestine.
Soluble dietary fiber is rapidly fermented by the microbiota of the final small intestine and the proximal large intestine.
It increases viscosity, reduces intestinal transit, and usually reduces consumption due to the satiety effect it produces.
Insoluble dietary fiber passes through the intestine without being completely digested, increasing intestinal transit depending on its size.
It increases fecal volume and only a small part is fermented since pigs lack the necessary enzymes for it.
The immunomodulatory effects of dietary fiber on pigs gut health are being studied due to their complexity. It could cause that certain components of dietary fiber (such as the inclusion of oligosaccharides and soluble fiber) would act either directly or indirectly as the growth promoters did.
The microbiota ferments soluble fiber components in the distal part of the small intestine and in the proximal part of the large intestine, leading to the formation of short chain fatty acids (SCFA), branched chain fatty acids (BCFA), lactate, amines, indole, phenolic compounds and gases such as hydrogen, carbon dioxide and methane [4].
In the absence of dietary fiber, the microbiota ferments the protein found in the large intestine.
This gives rise to compounds such as BCFA, ammonia (from the deamination of amino acids) that is absorbed and expelled in the form of urea and phenols (because of the decarboxylation of amino acids). It is toxic for pigs as it creates a dysbiosis in the intestine.
In the presence of fermentable carbohydrates, ammonia is expelled as part of the microbial biomass since it is used by the microbiota for its growth.
The production of SCFA, and more specifically of butyric, in the intestine improves intestinal health by maintaining an anaerobic environment, the immune system of pigs, and improves the function of the intestinal barrier by stimulating the differentiation of globet cells and the production of mucus [5] , [6]. SCFAs promote the differentiation and function of colon Treg cells [7] that maintain homeostasis by inhibiting the regulatory factor of their function and increasing the production of IL-10 that prevents inflammation (although this inflammation can sometimes help in the process of fighting pathogenic bacteria).
And in case of a dysbiosis?
In case of dysbiosis, homeostasis is lost because the facultatively anaerobic microbiota, such as Proteobacteria (E. coli and Salmonella), is developed at the expense of oxygen-sensitive butyrate-producing bacteria. Thus, we could say that the “disanabacteriosis” typical of a healthy intestine would pass into a microaerophilic environment with the development of a facultative anaerobic microbiota3 (Figure 3 modified from Jha R, 2019).
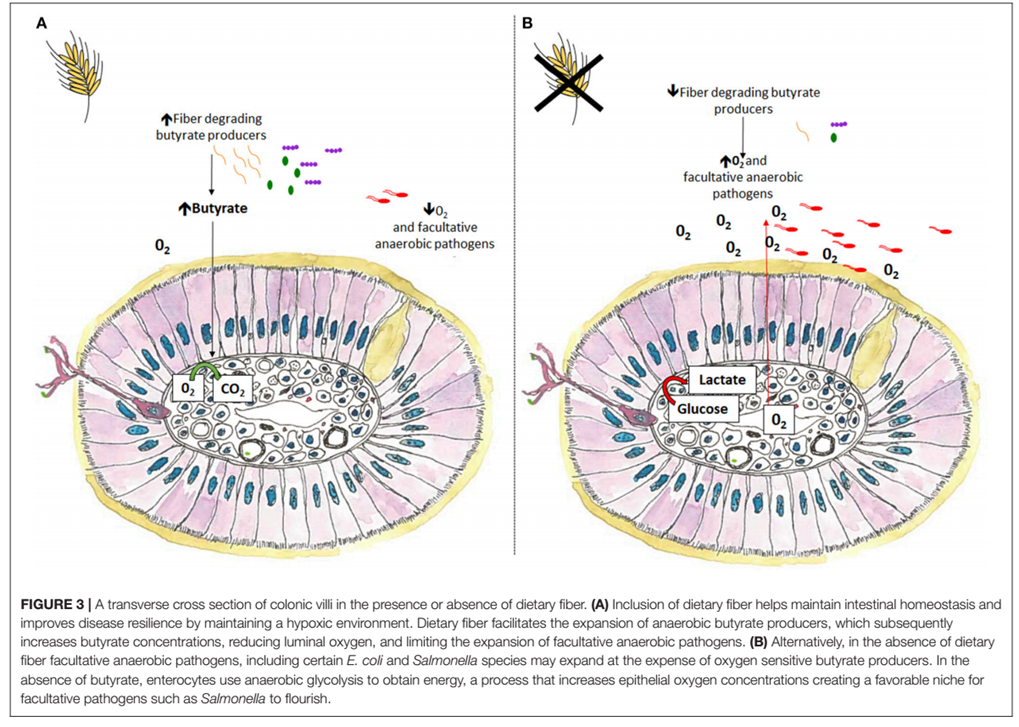
Figure 3: Cross section of the villi of the colon in the presence or absence of dietary fiber.
Furthermore, the energy provided by the SCFAs to the enterocytes is essential for their functioning. SCFAs promote the proliferation of the epithelium of the intestinal mucosa, as well as the height of the intestinal villi. The epithelium of the intestinal mucosa regulates nutrient exchanges, is also responsible for intestinal secretions rich in digestive enzymes and influences the adhesion capacity of bacteria to the intestine.
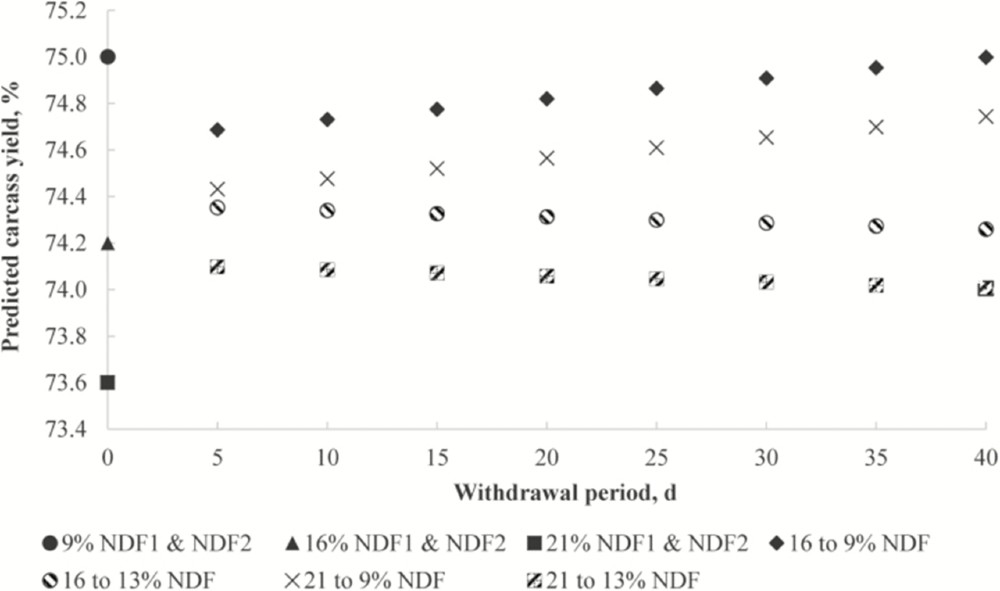
Dietary fiber (especially insoluble fiber) increases endogenous losses formed by desquamation epithelium, mucus and secretion products. It reduces the amount of energy and some nutrients absorbed. For this reason, It has been considered as an anti-nutritional factor. This characteristic is more pronounced in birds than in pigs, although it is true (demonstrated among others by Soto et al 2019) [8] that the levels of fiber, in this case of neutral detergent fiber (NDF), influence other characteristics such as channel performance.
Figure 5: Prediction of carcass performance depending on the levels of neutral detergent fiber and the fasting period in fattening pigs (Soto et al 2019) 4
The incorporation of soluble dietary fiber increases the viscosity of the diet (depending on the type of fiber), which can increase the atrophy of the intestinal villi. The ratio between the height of the villi and the depth of the crypts is an objective criteria to evaluate the digestive capacity of the small intestine. However, it is not a measure that should be standardized since it varies with the pig’s age, diet, and intestinal section in which we measure it.
The decrease in the speed of intestinal transit caused by the increase in viscosity causes certain bacteria to proliferate, which alterates the balance of the microbiota. Resistant starch also increases the viscosity of the diet, although unlike dietary fiber, the result of its metabolism gives compounds of lower molecular weight promoting the growth of different bacterial groups such as bifidobacteria and lactobacilli.
References
[1] Las células de Paneth son un tipo de células del epitelio del intestino delgado con funciones protectoras fundamentalmente. Se localizan en las criptas de Lieberkühn. Contienen gránulos con compuestos antimicrobianos y otros compuestos reguladores de la inmunidad para el mantenimiento de la barrera gastrointestinal.
[2] Conway P. Function and regulation of the gastrointestinal microbiota of the pig. In: Souffrant W, Hagemeister H, editors. Proceedings of the VIth International Symposium on Digestive Physiology in Pigs. Dummerstof (1994). p. 231–40.
Montagne L, Pluske J, Hampson D. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. (2003) 108:95–117.
Jha R, Fouhse JM, Tiwari UP, Li L, y Willing BP. 2019. «Dietary Fiber and Intestinal Health of Monogastric Animals». Frontiers in Veterinary Science 6.
[3] Hoffman, Rhonda M. 2013. «8 – Carbohydrates». In Equine Applied and Clinical Nutrition, edited by Raymond J. Geor, Patricia A. Harris, and Manfred Coenen, 156-67.
[4] Jha R, Berrocoso JFD. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim Feed Sci Technol. (2016) 212:18–26
[5] Wrzosek L, Miquel S, Noordine ML, Bouet S, Chevalier-Curt MJ, Robert V, et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. (2013) 11:61
[6] Las células caliciformes o células goblet, son células epiteliales especializadas o “glándulas unicelulares” secretoras de mucus, presentes en los revestimientos epiteliales de las mucosas de las vías respiratorias y el aparato digestivo. Las células caliciformes también secretan proteínas antimicrobianas, quimiocinas y citocinas que demuestran funciones en la inmunidad innata, más allá del mantenimiento de una barrera. La mucina es un mucopolisacárido, un material normalmente traslúcido y viscoso que se disuelve en agua para formar el moco. El moco protege a los enterocitos actuando como una barrera física frente a bacterias y otros patógenos como los parásitos intestinales. Contiene también grandes cantidades de anticuerpos como inmunoglubulina A (IgA) capaz de atrapar bacterias invasoras (https://es.wikipedia.org/wiki/C%C3%A9lula_caliciforme)
[7] Las células Treg son linfocitos T que regulan o suprimen a otras células del sistema inmunitario. Las células Treg controlan las respuestas inmunitarias de partículas extrañas o propias (los antígenos) y ayudan a prevenir enfermedades autoinmunes. Existen dos tipos principales: las producidas en el timo (Tregs “naturales” – nTreg o Tregs del timo, tTreg) o aquellas que se diferencian a partir de células T activadas en la periferia o en cultivos celulares (Tregs “adaptativas o inducidas” – iTreg). (http://inmunologia.eu/celulas-inmunologia-en-un-mordisco/celulas-t-reguladoras-tregs)
[8] Soto, Jose A, Mike D Tokach, Steve S Dritz, Márcio A D Gonçalves, Jason C Woodworth, Joel M DeRouchey, Robert D Goodband, Mariana B Menegat, y Fangzhou Wu. 2019. «Regression analysis to predict the impact of dietary neutral detergent fiber on carcass yield in swine». Translational Animal Science 3 (4): 1270-74.
Author: Morillo Alujas, Alberto
Artículo publicado en porciNews