Adverse reactions to veterinary drugs decreased in 2020 in Spain according latest report of AEMPS
According to the latest report published by the Spanish Agency for Medicines and Health Products (AEMPS) in its annual Veterinary Pharmacovigilance Bulletin, veterinary medicines showed a higher level of efficacy, safety and quality on 2020 compared to the previous period.
Every year, the AEMPS publishes all the activities carried out in the field of pharmacovigilance to prove whether the veterinary drugs in force are safe and effective for the animals. The objective is to verify that the Benefit / Risk (B / R) balance of the veterinary drugs maintains the margins that were exposed when their commercialization was approved. Thus, identifying new risks (or significant variations of whether the severity and / or frequency of those previously) is how we can face the associated dangers for public health, animal health and the environment.
Still, we should highlight the direct impact on the products safety the following aspects: human handling of the drug, biosecurity concepts, animals that are in contact, …
The AEMPS emphasize that not because a veterinarian drug is between the allowed margins, it can be qualified as “extraordinary”: never a product is totally harmless or free from potential adverse effects. And this is exactly the origin of this pharmacovigilance: to test that all product in force is being used according to what it is stablished as “safe”.
What does the AEMPS consider as Suspected Adverse Reaction?
Suspected adverse reaction means any adverse event for which there is a reasonable possibility that the drug is the causant of the adverse event. For the purposes of Investigational New Drug Application (IND), “reasonable possibility” implies there is enough evidence to suggest a causal relationship between the drug and the adverse event.
The AEMPS resumes the following ones:
Suspected adverse reactions (AR) in animals under normal (ie authorized) conditions of use of the product.
RS after use not contemplated (any variation with respect to what was established at the time of authorization of the veterinarian drug)
Lack of the expected efficacy
Insufficiency of the waiting time, causing a residual level above the Maximum Residue Limits (MRL).
Reactions in people when being in contact with the veterinarian drug or animals that have been treated with the product.
Environmental problems
Transmission of infectious agents
Individual notifications of Suspected Adverse Reaction Events
The AEMPS highlights that in Spain in 2020 there were 69 fewer Suspected Adverse Events than in 2019. Of the 1,941 events registered, 1,510 were cases reported for the first time.
Regarding these, 20 affected people and the target species in which they were presented were the following (in order from highest to lowest number): dog, cat, sheep, pig, cow, rabbit, goat, hen, horse, ferret, seabass, bee and rat.
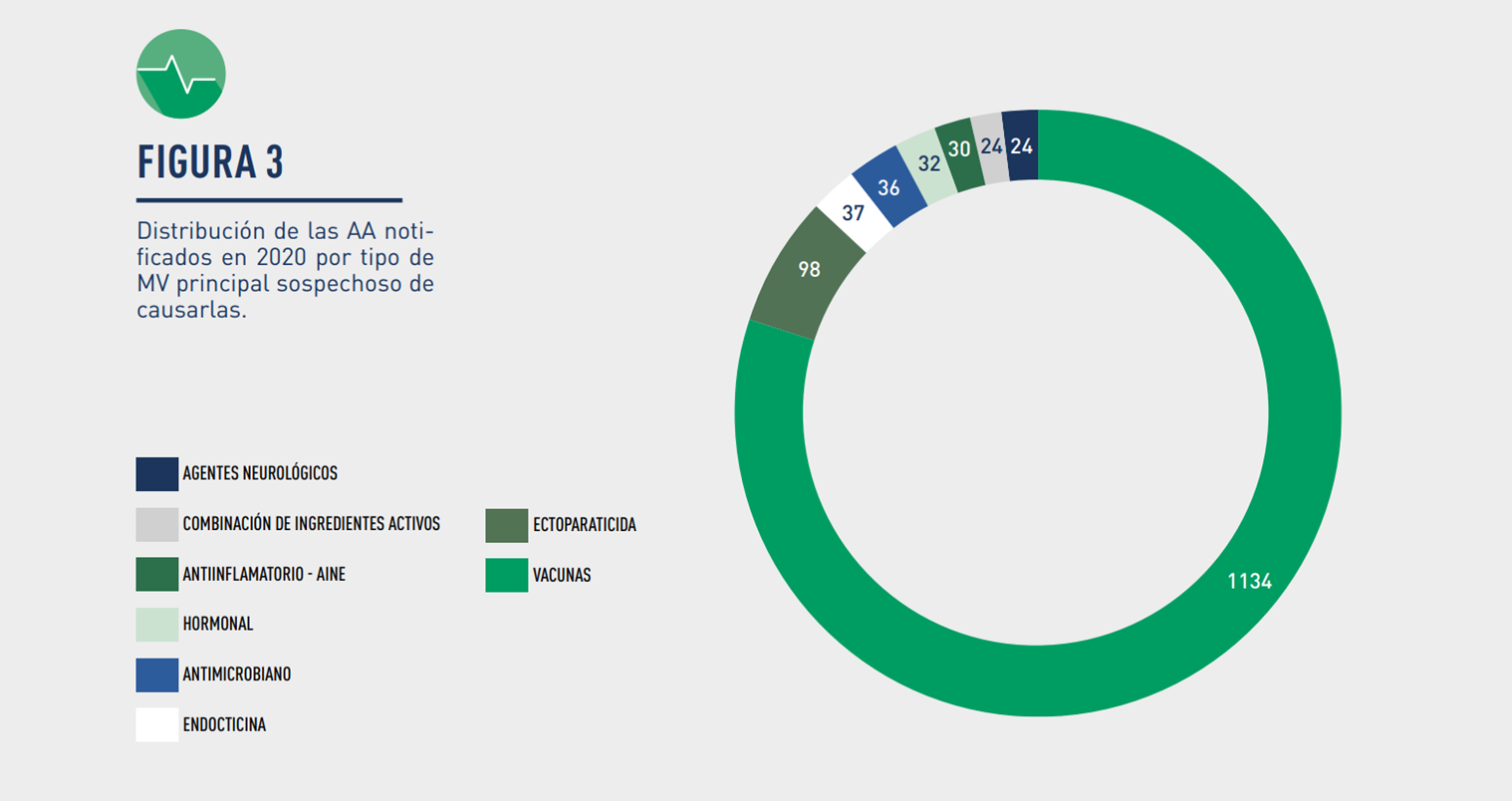
Image: Distribution of Suspected Adverse Events notified in 2020 by Veterinary Drug group.
The initial notifications were mostly made by the Holders. The rest of the sources of origin were health professionals, farmers, animal owners, other users, etc.
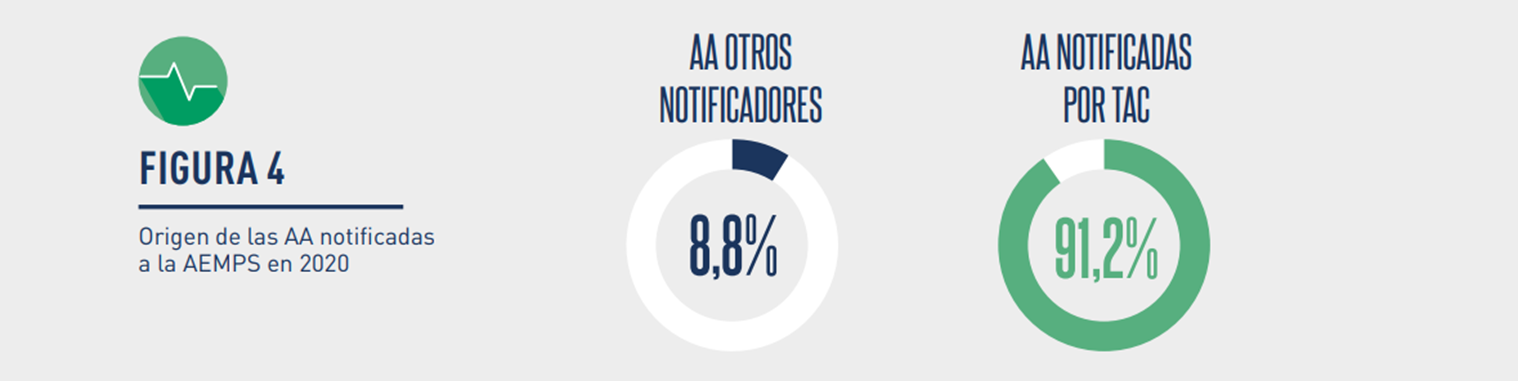
Image: Origin of the initial notifications
Alerts, Periodic Safety Reports and Post Authorization Studies
Regarding the Periodic Security Reports, a total of 1,700 were received.
37 of them concluded that it was necessary to proceed with the modification of the Technical Sheets.
Committee on Safety of Veterinary Medicines (CSMV in Spanish)
The Committee on Safety of Veterinary Medicines (CSMV) is in charge of technical and scientific advice regarding the pharmacovigilance of veterinarian drugs. In 2020, 3 meetings were held to discuss the modification of the marketing authorization of the 37 drugs that we mentioned above. Thus, and in accordance with the provisions, they published the individual proposal to modify the texts for each drug, which is available in their report.

Non-urgent safety information and alerts (NUIS) for pharmacovigilance reasons and quality defects.
The AEMPS also indicates the number of alerts and non-urgent safety reports (NUIS) for pharmacovigilance reasons, with the result of no cases decreed in 2020. 5 NUIS were received and managed and 6 alerts were registered for quality defects.
Promotion of pharmacovigilance
The AEMPS maintains a strategic focus on spreading the importance of Pharmacovigilance. The EU considers increasing notification levels as a priority, which is why this objective has been included in the strategic planning of the Group of Heads of EU Agencies (HMA) and in its Subgroup on Pharmacovigilance Strategies (ESS).
Participation in international meetings
In relation to the pharmacovigilance activities at the European level in 2020, it is worth highlighting the participation of the following Groups and Committees: CVMP Veterinary Pharmacovigilance Group, VEDDRA Terminology Subgroup, Regulators Group on the IPS Worksharing program and HMA ESS Subgroup .
Likewise, we should highlight the active participation of three selected experts in the acts of development of Regulation (EU) 2019/6 of the European Parliament and of the Council of December 11, 2018 on veterinary drugs and by which Directive 2001/82 is repealed /EC.
At Tests&Trials, we are specialized in this field and for more than 20 years have led more than 100 projects to verify the safety, efficacy and quality of the veterinarian drugs. Contact us and we will be delighted to help you with your business needs.